Batteries Notes...
Page Contents
References
- Electron Activity in Chemical Reactions, Chapter 11 - Batteries And Power Systems, All About Circuits
- Battery Ratings, Chapter 11 - Batteries And Power Systems, All About Circuits
- Ionic vs Covalent Bonds - Understand the Difference, ThoughtCo.
- Why does the battery voltage change?, buildElectronicCircuits.com.
- How to calculate battery run-time, PowerStream, July 31, 2019.
Chemistry Of Batteries
Chemical Bonds
Covelant bond (a.k.a. molecular bond): Atoms share electrons in their outer orbitals. Weaker bond than ionic bonds. Only form between non metals.
Ionic bond: Atom "donates" electron(s) to its "partner": electron spends most of its time close to the bonded atom. Much stronger than a covelant bond. Can form between metals or nonmetals.
Battery Cells
Battery cells use ionic solutions because the transfer of electrons between atoms can generate electric current:
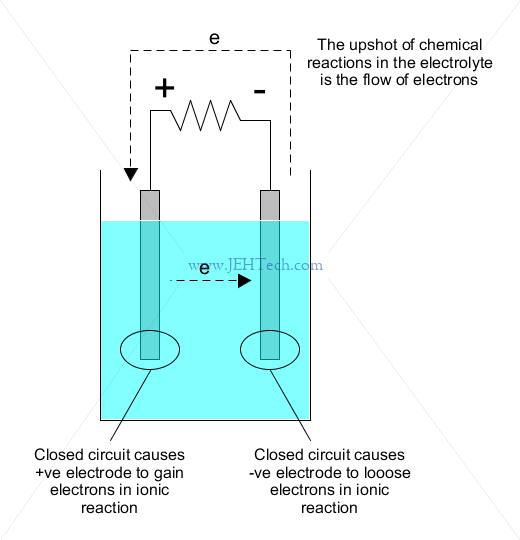
Once all molecules in the electrolyte have been "used up", meaning there are no more molecules left to react with the positive electrode, the battery is depleted.
Some electrolytes can be rechared by an external source connected in the opposite direction so that it essentially "undoes" the reaction caused during discharge.
Batteries are not ideal - they have an internal resistance. Cells are connected in parallel to minimise this.
A primary cell is rechargable. A secondary cell is not.
Battery Lifetime
Measured in amp-hours. Capacity of 1 amp-hour means battery should be able to supply a current of 1 amp for an hour, 2 amps for half an hour etc. It is a unit that describes available charge of a battery (see last paragraph).
Amp-hours normally specified at a given current, time or assumed 8-hours. Done because the discharge profile of a battery will vary with current draw. E.g. if battery has a 2 amp-hour capacity specified for a current of 2 amps, then it means the battery can suuply a current of 2 amps for 1 hour ( 2 amp-hour / 2 amps).
Discharge profile also changes with the age of the battery, temperature and the remaining charge.
As batteries discharge their internal resistance also increases. Thus, voltage of battery should be checked under load to get correct reading.
$$ \text{amp-hour} = \text{continuous current (amps)} * \text{discharge time (hrs)} $$ Therefore, an estimate of battery life can be made if the average current draw is known: $$ \text{discharge time etc. (hrs)} = \frac{\text{amp-hour}}{\text{avg continuous current (amps)}} $$
Coulomb counters monitor the number of Coulombs (defined as $6.25 \times 10^{18}$ electrons) seen coming out of a battery (or in if recharging). They rarely give the value in Coulombs. 1 amp is 1 Coulomb per second past a point in a circuit - current is speed of charge flow around a circuit. In an hour there are 3600 seconds, so 1 amp-hour means 3600 coulombs. The counters will normally tell the total discharge seen so far in amp-hours or some similar unit.